
Who We Are
A business unit of the New York Blood Center Enterprises, Comprehensive Cell Solutions (CCS) is a cell and gene therapy-focused development and manufacturing organization that provides critical capacity to an underserved population of early-stage developers, as well as expertise and strategic footprint for hospital treatment centers and pharmaceutical organizations.
What We Do
- Cell Sourcing and Apheresis
- HLA Testing
- Process Development
- Analytical Development
- Platform-agnostic cGMP and GTP
- Fill/Finish
- Cryopreservation Center of Excellence
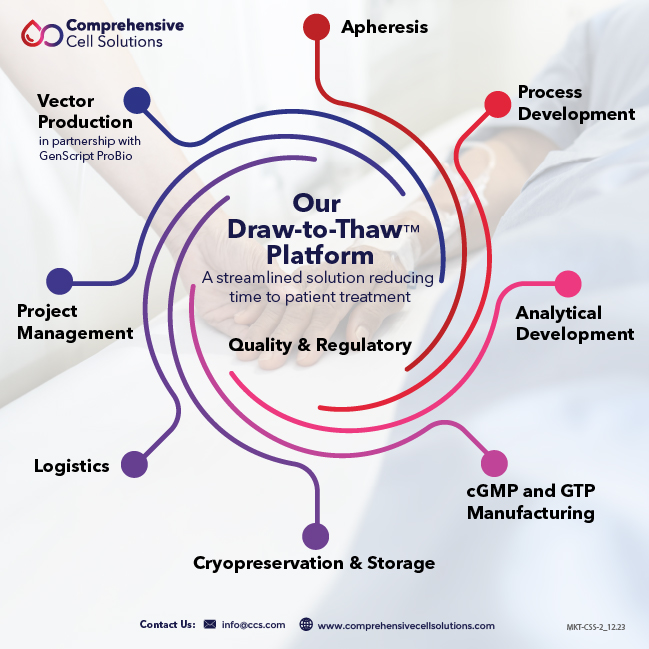
With a 50-year history of excellence in blood operations, collection facilities in 17 states, and partnerships with major hospitals, CCS offers a true end-to-end solution for our cell therapy clients. We call this our “draw to thaw” platform, a unique offering that begins and ends with the patient, accelerating the time from apheresis to treatment.

Their emphasis on innovative technology, extreme quality, and shorter timelines make them an excellent partner to fulfil our clients’ needs for lentiviral vectors, plasmids, linearized DNA and IVT-mRNA.
CCS is proud to work with GenScript ProBio, leaders in the development and manufacture of viral and non-viral vectors.
 With the patients we serve at the center of our efforts, our Cryopreservation Center of Excellence provides hospital, biotech, and pharma clients with the industry’s gold standard in cryopreservation services for cellular products, tissues, and biomaterials.
With the patients we serve at the center of our efforts, our Cryopreservation Center of Excellence provides hospital, biotech, and pharma clients with the industry’s gold standard in cryopreservation services for cellular products, tissues, and biomaterials.
Beginning with our Flagship Center of Excellence in Manhattan, we will continue to partner and establish key alliances with industry and academic experts to advance best practices, expand our footprint, identify new technologies and educate the next generation of biomanufacturing professionals.
- 60,000 units of cryopreserved cord blood
- 20 years of viability data
- 18,000 cell and tissue products
- 6,000 bone marrow transplants
Why CCS?
For more than 20 years, CCS has been pioneering advances in the research, development, and manufacture of cell therapies:
- Unmatched procurement of apheresis and cord blood products
- Access to our expansive network of NYBCe services
- Robust process and analytical development
- Regulatory consulting across all phases of development
- Skilled staff and ready capacity at our accredited facilities
- Collaborative, end-to-end partners
